CDSCO Flags Paracetamol and Pan-D in List of Failed Drugs, Check Details Here
New Delhi, 27th September 2024: In a recent quality assessment conducted by the Central Drugs Standard Control Organization (CDSCO), 53 medicines were found to have failed the safety and efficacy standards. The list includes commonly used medications for conditions such as high blood pressure, diabetes, acid reflux, and vitamin supplements. Several well-known pharmaceutical companies’ products, including widely prescribed drugs like paracetamol and diclofenac, have been flagged as unsafe for consumption.
Among the medicines that failed the test are key treatments like paracetamol, a common fever reducer, and diclofenac, used for pain relief. Additionally, antifungal drugs like fluconazole and several others manufactured by leading pharmaceutical firms have also been classified as harmful.
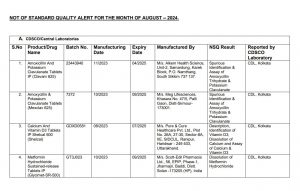
Of the 53 failed medicines, the names of only 48 have been confirmed. The manufacturers of five of the failed drugs have claimed that counterfeit versions of their products are being sold under their brand names. One notable failure includes the Pantocid Tablet, produced by Sun Pharma.
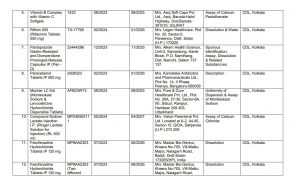
Among the failed drugs are treatments that have seen increased use over the years. Common medications like Amoxicillin and Potassium Clavulanate (Calvum 25 and Maxclav 625), Metformin Hydrochloride (Glycimet-SR-500), and Pantoprazole (Pan-D) are on the list. These are often prescribed for infections, diabetes, and acid reflux, respectively. Other failed medications include Ringer Lactate Solution for Injection, Ibuprofen and Paracetamol Oral Suspension, Fexofenadine Hydrochloride Tablets, and various calcium and vitamin D3 supplements.
Medications like Pantocid, used for treating acidity, are commonly prescribed, and the failure of such a drug is concerning given its widespread use. Drugs like Shelcal 500, a calcium and vitamin D3 supplement, are also among those that did not meet quality standards.
The central government has warned consumers against using these failed medicines, citing potential health risks. They issued a specific caution about enzyme-based medicines containing glucoamylase, pectinase, amylase, protease, and bromelain, which were found to be unsafe. The list also includes anti-parasitic drugs used for hair treatments, which have also failed the CDSCO quality test.
The complete list of failed drugs includes commonly used treatments such as:
Amoxicillin and Potassium Clavulanate Tablets IP (Calvum 25)
Amoxicillin and Potassium Clavulanate Tablets (Maxclav 625)
Calcium and Vitamin D3 Tablets IP Shelcal 500 (Shelcal)
Metformin Hydrochloride Sustained-Release Tablets IP (Glycimet-SR-00)
Metformin Hydrochloride Sustained-Release Tablets IP (Glycimet-SR-500)
Vitamin B Complex with Vitamin C Softgels
Rifamin 550 (Rifaximin Tablets 550 mg)
Pantoprazole Gastro-Resistant and Domperidone Prolonged-Release Capsules IP (Pan-D)
Paracetamol Tablets IP 500 mg
Montair LC Kid (Montralukast Sodium and Levocetirizine Hydrochloride Dispersible Tablets)
Compounded Sodium Lactate Injection I.P(Ringer Lactate Solution for Injection) (RL 500ml)
Fexofenadine Hydrochloride Tablet IP 120 mg
Fexofenadine Hydrochloride Tablet IP 120 mg
Laxanorm Solution (Lactulose Solution USP)
Heparin Sodium Injection 5000 Units (Hostranil Injection)
Buflam Forte Suspension (Ibuprofen and Paracetamol Oral Suspension)
Cepodem XP 50 Dry Suspension (Cefpodoxime Proxetil and Potassium Clavulanate Oral Suspension)
Nimesulide, Paracetamol and Chlorzoxazone Tablet (NICIP MR)
Rolled Gauze (Non-sterilized)
Ciprofloxacin Tablet I.P. 500 mg (Osif-500)
Nimesulide, Phenylephrine Hydrochloride and Levocetirizine Dihydrochloride Tablet (Nunim-Cold)
Adrenaline Injection I.P. Sterile 1 ml
Compound Sodium Lactate Injection I.P. (Ringer Lactate Solution for Injection) RL 500 ml
Wingel XL Pro Gel (Diclofenac Diethylamine, Linseed Oil, Methyl Salicylate and Menthol Gel)
Atropine Sulphate Injection I.P. 2 ml
Cefoperazone and Sulbactam for Injection (Tudecef 1.5 gm)
Heparin Sodium Injection I.P. 25000 IU/5 ml
Cefepime and Tazobactam for Injection (Cruapime-TZ Kid Injection)
Atropine Sulphate Injection I.P. (Atropine Sulphate)
Atropine Sulphate Injection I.P. (Atropine Sulphate)
Atropine Sulphate Injection I.P. (Atropine sulfate)
Atropine sulfate injection I.P. (Atropine Sulphate)
Salbutamol, Bromhexine HCI, Guaifenesin and Menthol Syrup (Ecogyl Expectorant)
Diclofenac Sodium IP
Escitalopram and Clonazepam Tablets IP (Clozaps-ES Tablet)
Phenytoin Sodium Injection USP
Paracetamol, Phenylephrine Hydrochloride and Cetirizine Hydrochloride Suspension (Cethal Cold DS Suspension)
Calcium 500 mg with Vitamin D3 250 IU Tablets IP
Amoxicillin and Potassium Clavulanate Tablets IP 625 mg (Renamega-CV 625)
Olmesartan Medoxomil Tablets IP 40 mg
Infusion Set-NV
Telmisartan Tablets IP 40 mg
Telmisartan Tablets IP 40 mg
Telmisartan Tablets IP 40 mg
Metronidazole Tablets IP 400 mg
Alprazolam Tablets IP 0.25 Migra (Erazol-0.25 Tablet)
Glimepiride Tablet IP (2 mg)
Calcium and Vitamin D3 Tablet IP
Several injectable medications and treatments for various ailments have also been flagged, and authorities are urging consumers to consult their healthcare providers before continuing the use of these drugs.
The government is now investigating the matter further and has emphasized the importance of stringent regulation to prevent the circulation of counterfeit and harmful drugs in the market.








